 |
TECENTRIQ combination* is now approved for 1L
|
|---|
Dear Colleagues,
TECENTRIQ, in combination with Avastin (bevacizumab) and carboplatin/paclitaxel (carbo/pac) is a new regimen indicated for 1L treatment of mNSCLC. TECENTRIQ combination* is the first cancer immunotherapy to demonstrate meaningful OS advantage in EGFR/ALK+ who have previously progressed on tyrosine kinase inhibitors (TKIs) as well as in patients with liver metastases (mets). The efficacy and safety of TECENTRIQ combination* was established in the IMpower150 clinical trial.
IMpower150
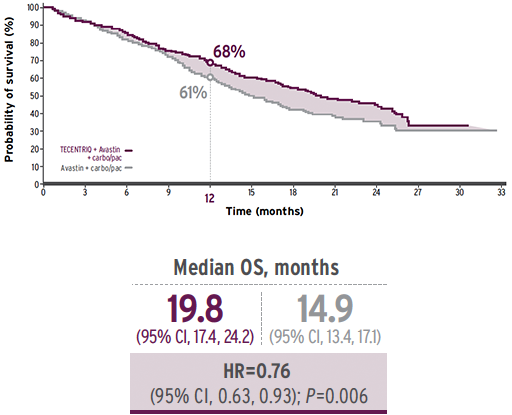
A phase III multicentre, international, randomised, open-label, 3-arm
trial in mNSCLC (N=1,202) comparing TECENTRIQ+Avastin+carbo/pac vs Avastin+carbo/pac alone. TECENTRIQ combination*: delivered a statistically significant OS benefit vs Avastin + carbo/pac alone |
 |
HR= Hazard ratio
CI = Confidence interval
PFS at 12 months was nearly doubled in TECENTRIQ combination*
vs Avastin+carbo/pac alone (38% vs 20%, HR= 0.59;95% CI, 0.69; P<0.0001). TECENTRIQ combination* is the first cancer immunotherapy combination to demonstrate clinically meaningful survival in EGFR/ALK+ who have progressed on TKIs as well as in patients with liver mets.
Median OS (95% CI, 0.29, 1.03) was NR in TECENTRIQ combination* vs 17.5 months in Avastin + carbo/pac. Median OS (95% CI, 0.33, 0.82) in liver mets was 13.3 months versus 9.4 months in Avastin + carbo/pac.
HR= Hazard ratio
A majority of patients responded to TECENTRIQ combination* vs Avastin+carbo/pac alone |
 |
HR= Hazard ratio
CI = Confidence interval
ORR was 56.4% (95% CI, 51.4, 61.4) with TECENTRIQ combination* vs 40.2% (95% CI, 35.3, 45.2) with Avastin+carbo/pac.
|
 |
VIDEO
During ESMO 2018, Prof. Martin Reck, MD, PhD (Principal
investigator of the IMpower150 trial) from Grosshansdorf Lung Clinic, Grosshansdorf, Germany provided a summary of the trial design and its key results. |
 |
This video has been produced and funded by VJ Oncology. F. Hoffmann-La Roche Ltd did not participate in the filming or editing of this video. The video reflects independent opinions of the speaker.
|
Test your knowledge on TECENTRIQ and cancer immunotherapy
Take the quiz and find out how much you know about TECENTRIQ clinical trials and cancer immunotherapy
|
Full prescribing information available.
Download the EU TECENTRIQ SmPC here. |


Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου