Distal Cauda equina syndrome: A case report of lumbosacral disc pathology and review of literature
Category: Neuropathology
Article Type: Case Report
Michael J. Benko, Aaron P. Danison, Eric A. Marvin, Brian F. Saway
Division of Neurosurgery, Virginia Tech Carilion School of Medicine Roanoke, Virginia, United States.
DOI:10.25259/SNI-152-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
How to cite this article: Michael J. Benko, Aaron P. Danison, Eric A. Marvin, Brian F. Saway. Distal Cauda equina syndrome: A case report of lumbosacral disc pathology and review of literature. 10-May-2019;10:84
How to cite this URL: Michael J. Benko, Aaron P. Danison, Eric A. Marvin, Brian F. Saway. Distal Cauda equina syndrome: A case report of lumbosacral disc pathology and review of literature. 10-May-2019;10:84. Available from: http://surgicalneurologyint.com/surgicalint-articles/distal-cauda-equina-syndrome-a-case-report-of-lumbosacral-disc-pathology-and-review-of-literature/
Date of Submission
02-Dec-2018
Date of Acceptance
25-Feb-2019
Date of Publication
10-May-2019
Page views
127
Steiner SkyscraperElekta SkyscraperSteiner SkyscraperElekta Skyscraper
Abstract
Background: Cauda equina syndrome (CES) is an uncommon entity that presents acutely with all or some of the following symptoms; urinary incontinence from retention, fecal incontinence from loss of sphincter tone, saddle area hypoesthesia or anesthesia, and acute or progressive weakness in one or both lower extremities. The protean symptomatology is often mixed and is vulnerable to confounding comorbidities making the accurate and timely diagnosis of this syndrome uniquely challenging. Here, we present the case of a man who developed isolated sacral nerve dysfunction from CES in the midst of a diabetic crisis.
Case Description: A 53-year-old male with a long history of uncontrolled Type 2 diabetes presented with acute-onset urinary and fecal incontinence, scrotal anesthesia, and a 3-day history of lower back pain with intermittent bilateral leg pain. This patient displayed no objective changes in leg strength, sensation, or reflexes. In addition, the patient tested positive for cocaine and had a blood glucose level of 800 mg/dL which confounded his clinical picture. The patient underwent bilateral laminectomies from L4–S1 with the removal of a large sequestered disc fragment from the S1–S2 disc space within 8 h of presentation with reasonable recovery.
Conclusion: Highly variable presentations often confound the accurate and timely diagnosis of CES with severe implications on quality of life. Despite the limited functional recovery seen after surgical decompression, urgent or emergent intervention is paramount for treatment. Our patient’s presenting symptomatology and comorbidities highlight the need for practitioners to maintain a high index of suspicion in anyone with incontinence and back pain, regardless of distractors and even in the absence of other anticipated motor or sensory findings.
Keywords: Cauda, equina, herniation, neurosurgery, syndrome
INTRODUCTION
Cauda equina syndrome (CES) is an uncommon, but well-known surgical entity that is often seen in primary care clinics and emergency departments. It characteristically presents acutely with some or all of the following symptoms: urinary retention with or without overflow incontinence, fecal incontinence from loss of anal sphincter tone, saddle area hypoesthesia or anesthesia, and acute or progressive weakness in the muscles of one or both lower extremities.[ 17 , 27 , 28 ] Although the time frame for surgical decompression has not been clearly delineated, it is often considered a surgical emergency and the presence of a true CES is typically treated within 24–48 h if possible.[ 3 , 13 , 51 ] The incidence of CES is approximately 0.04% (1:2500) in all patients who present with low back pain and only 1–2% of patients with lumbar disc herniations which is the most common cause, though the etiology of CES is diverse and can be caused by tumor, trauma, and infections.[ 1 , 2 , 5 , 6 , 18 , 21 , 24 , 25 , 33 , 39 , 43 , 45 , 50 , 52 ] The documented incidence appears to be on the rise, however, and given the legal implications surrounding the diagnosis and misdiagnosis of this entity, the true clinical syndrome may be overreported in literature.
Despite CES being well established among spine surgeons, there are several variables that can often contradict the suspected diagnosis and lead to delays in treatment. These can include inconsistent portions of the reported history, the physical examination, and even lumbar imaging.[ 7 ] Given the physical and legal ramifications, ruling out CES becomes the clinical responsibility of the treating physician which can be both challenging and fraught with peril. We suggest that a high level of suspicion should be maintained when caring for any patient presenting with urinary or fecal incontinence and acute or chronic back pain, even in the absence of other associated motor or sensory findings. We now report an atypical presentation of distal CES caused by a large, sequestered, caudally migrated L5–S1 herniated disc fragment.
CASE REPORT
A 53-year-old male with a long history of uncontrolled Type 2 diabetes presented to the emergency department after recent cocaine ingestion with newly discovered scrotal numbness and several episodes of urinary and fecal incontinence. His finger-stick blood glucose was measured to be 800 mg/dL before consultation and his hemoglobin A1C was >18.5%. He endorsed mild-moderate low back pain for the previous 3 days with intermittent bilateral leg pain that was nondermatomal in distribution but denied any leg weakness or difficulty ambulating. His physical examination confirmed that his motor strength was full and his sensation to light touch was intact in all lower extremity muscle groups and tested dermatomes bilaterally. He also had normal patellar and Achilles deep tendon reflexes bilaterally. He did have perineal anesthesia to light touch and pinprick testing, as well as poor rectal tone. The patient had already received a Foley catheter before consultation without precatheter bladder volume assessment or documented initial urinary output.
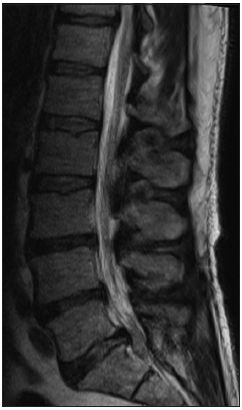
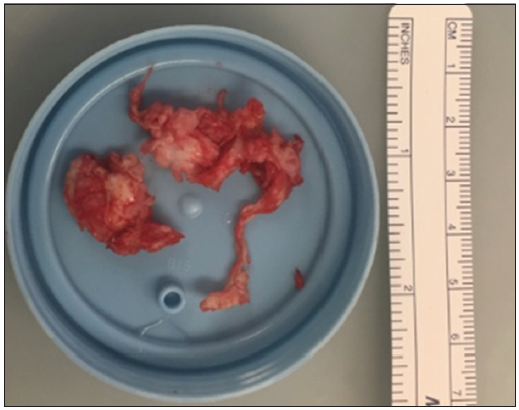
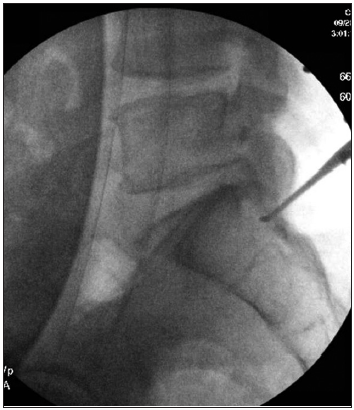
He underwent an emergent lumbar magnetic resonance imaging which demonstrated mild-moderate multilevel degenerative spondylosis in the setting of congenital canal stenosis with significant lateral recess stenosis at L4–5 and a centrally herniated nucleus pulposus (HNP) at L5–S1 [ Figure 1 ]. After discussing the risks and benefits of surgical decompression, the patient elected to proceed and was brought to the operating room for surgery within 8 h. Bilateral posterior laminectomies were performed at L4 and L5. The patient was noted to have severe stenosis caudal to the S1 lamina intraoperatively, so this was also removed bilaterally and revealed a large sequestered herniated disc fragment [ Figure 2 ] posterior to the S1–S2 disc space [ Figure 3 ] causing severe sacral nerve compression. Although the fragment was not sent for microscopic evaluation, it was of typical appearance, texture, and size of an HNP, and there was a clear annular defect at L5–S1 consistent with its presumed origin. The patient did well after surgery with regard to his back pain, and his perineal sensation and bowel continence improved significantly during his hospitalization. He was discharged home with a Foley catheter and outpatient urology follow-up on postoperative day 5.
Figure 1
Lumbar magnetic resonance imaging demonstrating mild- moderate multilevel degenerative disease in the setting of congenital canal stenosis with significant lateral recess stenosis at L4–5 and a herniated nucleus pulposus at L5–S1.
Figure 2
Sequestered herniated disc fragment.
Figure 3
Radiograph demonstrating herniated disc fragment posterior to the S1–S2 disc space.
DISCUSSION
First described by Mixter and Barr, in 1934, the most common etiology of C. equina compression is an HNP.[ 36 ] Symptoms are a direct result of nerve root dysfunction and most authors would agree that micturition impairment should be present to diagnose CES.[ 13 , 30 , 41 , 47 ] Symptoms that support the diagnosis of CES include decreased anal sphincter tone, reduced sensation in the sacral dermatomes (saddle area), and sexual dysfunction.[ 3 , 5 , 28 , 35 , 42 ] It should be noted that symptoms of sciatica (neurological deficits in one or both lower extremities including changes in reflexes, motor function, or sensation) are more common[ 27 , 28 ] at the time of CES presentation and the prevalence of it has been quoted to be 97%, while micturition dysfunction was seen in 92%, saddle anesthesia in 93%, and defecation dysfunction found in 74% of patients.[ 28 ] However, many studies have identified discrepancies between physical examination findings, imaging findings, and accurate diagnosis highlighting the protean presentation and need for vigilance.[ 8 , 16 , 20 , 40 , 45 ]
It is estimated that fewer than 1 in 2000 of patients who present with acute severe lower back pain will be diagnosed with CES[ 49 ] and up to 19–41% of patients who are referred for CES evaluation have essentially negative imaging studies.[ 7 , 8 ] In fact, patients presenting to the emergency department with isolated low back pain rarely require urgent or emergent neurosurgical intervention,[ 11 , 14 , 31 ] yet there is a myriad of CES causes that can present with subacute or vague pain and symptomatology. Some well-documented etiologies include lesions such as intracanalicular hemorrhages,[ 12 , 25 ] tumors,[ 26 , 44 , 48 ] cysts,[ 37 , 53 ] lipomatosis,[ 52 ] infection,[ 1 ] and even perineural spread of endometriosis.[ 46 ] Moreover, inflammatory and degenerative conditions that lead to stenosis such as ankylosing spondylitis,[ 29 ] Paget’s disease,[ 39 ] osteoporotic stress fracture,[ 38 ] and hypertrophic degenerative stenosis[ 24 ] have also been well recognized in literature. While most pathologies have distinct imaging characteristics, there are various neoplastic causes of CES that has masqueraded as simple herniated discs including Ewing sarcoma,[ 22 ] osteosarcoma,[ 9 ] and even intradural metastatic cancer.[ 48 ] The vast etiological umbrella that underlies CES warrants a thorough workup on a case-by-case basis and should any doubt exist regarding intraoperative findings during the requisite surgical intervention, we would advocate that a specimen be provided for pathological analysis to confirm the etiology.
Despite CES being considered a surgical emergency with decompression often occurring in an expedient fashion, the postoperative resolution of deficits is historically quite poor.[ 19 ]
A meta-analysis by Korse et al., in 2017, found that at postoperative follow-up, the prevalence of residual sciatica was 48%, micturition dysfunction was 48%, altered saddle sensation was 57%, defecation dysfunction was 42%, and sexual dysfunction was 53%.[ 28 ] This emphasizes the point that assessing patients with this constellation of symptoms must be taken seriously as the impact on long-term quality of life is quite profound even with proper treatment. Moreover, comorbidities such as hyperglycemic neuropathy in a diabetic may have elements of these symptoms at baseline and can act as a distractor in cases of suspected CES as in our patient.[ 15 ] Indeed, CES diagnosis often becomes a mounting medicolegal issue with at least one study documenting that in 96% of cases of CES brought to a malpractice insurance organization, 65% progressed to claims by the affected patients, and 48% of these cases resulted in payment of compensation for damages.[ 34 ] It must be noted that rare cases of CES resulting from iatrogenic causes such as retained surgical cottonoids,[ 4 ] epidural steroid injections,[ 10 ] and even a pathological engorgement of the epidural venous plexus after elective lumbar microdiscectomy[ 32 ] have been reported in literature with additional consequences. Furthermore, studies have shown that a delay in treatment typically results in adverse legal decision highlighting the requisite expedited intervention.[ 19 , 23 ]
CONCLUSION
As in our patient with clinical distractors and undocumented bladder dysfunction before consultation, the diagnosis of CES often becomes difficult to specify and confirm, so we ultimately advocate for physicians to maintain a high index of suspicion for CES in any patient presenting with concern for sacral nerve dysfunction.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patients understand that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal N, Shah J, Hansberry DR, Mammis A, Sharer LR, Goldstein IM. Presentation of Cauda equina syndrome due to an intradural extramedullary abscess: A case report. Spine J. 2014. 14: e1-6
2. Ahn H, Jhaveri S, Yee A, Finkelstein J, Ahn H, Jhaveri S. Lumbar vertebral hemangioma causing Cauda equina syndrome: A case report. Spine. 2005. 30: E662-4
3. Ahn UM, Ahn NU, Buchowski JM, Garrett ES, Sieber AN, Kostuik JP. Cauda equina syndrome secondary to lumbar disc herniation: A meta-analysis of surgical outcomes. Spine (Phila Pa. 1976). 2000. 25: 1515-22
4. Akhaddar A, Baallal H, Elktaibi A. Abscess due to textiloma (gossypiboma: Retained surgical cottonoid). Surg Neurol Int. 2018. 9: 70-
5. Andersen JT, Bradley WE. Neurogenic bladder dysfunction in protruded lumbar disk and after laminectomy. Urology. 1976. 8: 94-6
6. Bal S, Kurtulmuş Ş, Koçyiğit H, Gürgan A. A case with Cauda equina syndrome due to bacterial meningitis of anterior sacral meningocele. Spine (Phila Pa. 1976). 2004. 29: E298-9
7. Balasubramanian K, Kalsi P, Greenough CG, Seetharam MP. Reliability of clinical assessment in diagnosing Cauda equina syndrome. Br J Neurosurg. 2010. 24: 383-6
8. Bell DA, Collie D, Statham PF. Cauda equina syndrome-what is the correlation between clinical assessment and MRI scanning?. Br J Neurosurg. 2007. 21: 201-3
9. BeşkonaklI E, Çayli SR, Okay Ö, Bostanci U, Ergün R. Pelvic osteosarcomas causing lumbosacral nerve root compression presenting as herniated intervertebral disc: Report of two cases. Neurosurg Rev. 2000. 23: 165-7
10. Bilir A, Gulec S. Cauda equina syndrome after epidural steroid injection: A case report. J Manipulative Physiol Ther. 2006. 29: 492.e1-e3
11. Carragee EJ, Hannibal M. Diagnostic evaluation of low back pain. Orthop Clin North Am. 2004. 35: 7-16
12. Cordan T, Bekar A, Yaman O, Tolunay Ş. Spinal subarachnoid hemorrhage attributable to schwannoma of the Cauda equina. Surg Neurol. 1999. 51: 373-5
13. DeLong WB, Polissar N, Neradilek B. Timing of surgery in Cauda equina syndrome with urinary retention: Meta-analysis of observational studies. J Neurosurg Spine. 2008. 8: 305-20
14. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain?. J Am Med Assoc. 1992. 268: 760-5
15. Dolman CL. Morbid anatomy of diabetic neuropathy. JAMA. 1963. 183: 225-
16. Domen PM, Hofman PA, Van Santbrink H, Weber WE. Predictive value of clinical characteristics in patients with suspected Cauda equina syndrome. Eur J Neurol. 2009. 16: 416-9
17. Fraser S, Roberts L, Murphy E. Cauda equina syndrome: A literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009. 90: 1964-8
18. Gitelman A, Hishmeh S, Morelli BN, Joseph SA, Casden A, Kuflik P. Cauda equina syndrome: A comprehensive review. Am J Orthop (Belle Mead NJ). 2008. 37: 556-62
19. Gleave JR, MacFarlane R. Cauda equina syndrome: What is the relationship between timing of surgery and outcome?. Br J Neurosurg. 2002. 16: 325-8
20. Gooding BW, Higgins MA, Calthorpe DA. Does rectal examination have any value in the clinical diagnosis of Cauda equina syndrome?. Br J Neurosurg. 2013. 27: 156-9
21. Hamdan A, Strachan RD, Nath F, Coulter IC. Counting the cost of negligence in neurosurgery: Lessons to be learned from 10 years of claims in the NHS. Br J Neurosurg. 2015. 29: 169-77
22. Hsieh CT, Chiang YH, Tsai WC, Sheu LF, Liu MY. Primary spinal epidural ewing sarcoma: A case report and review of the literature. Turk J Pediatr. 2008. 50: 282-6
23. Hussain SA, Gullan RW, Chitnavis BP. Cauda equina syndrome: Outcome and implications for management. Br J Neurosurg. 2003. 17: 164-7
24. Joffe R, Appleby A, Arjona V. Intermittent ischaemia of the Cauda equina due to stenosis of the lumbar canal. J Neurol Neurosurg Psychiatry. 1966. 29: 315-8
25. Kebaish KM, Awad JN. Spinal epidural hematoma causing acute Cauda equina syndrome. Neurosurg Focus. 2004. 16: e1-
26. Kim DY, Lee JK, Moon SJ, Kim SC, Kim CS. Intradural spinal metastasis to the Cauda equina in renal cell carcinoma: A case report and review of the literature. Spine (Phila Pa. 1976). 2009. 34: E892-5
27. Korse NS, Jacobs WC, Elzevier HW, Vleggeert-Lankamp CL. Complaints of micturition, defecation and sexual function in Cauda equina syndrome due to lumbar disk herniation: A systematic review. Eur Spine J. 2013. 22: 1019-29
28. Korse NS, Pijpers JA, van Zwet E, Elzevier HW, Vleggeert-Lankamp CL. Cauda equina syndrome: Presentation, outcome, and predictors with focus on micturition, defecation, and sexual dysfunction. Eur Spine J. 2017. 26: 894-904
29. Lo C, Nair KP, Romanowski CA, Fawthorpe FW, Lindert RB. Horse’s tail in bamboo spine: The ‘Cauda equina syndrome in ankylosing spondylitis. Pract Neurol. 2014. 14: 418-21
30. Ma B, Wu H, Jia L, Yuan W, Shi G, Shi J. Cauda equina syndrome: A review of clinical progress. Chin Med J (Engl). 2009. 122: 1214-22
31. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017. 389: 736-47
32. Maki Y, Takayama M, Hayashi H, Yokoyama Y, Agawa Y. Cauda equina syndrome due to dural sac shift with engorgement of the epidural venous plexus: Rare complication after lumbar microdiscectomy. World Neurosurg. 2017. 104: e15-8
33. Maloney P. Handbook of neurosurgery. Yale J Biol Med. 2008. 81: 210-1
34. Markham DE. Cauda equina syndrome: Diagnosis, delay and litigation risk. Curr Orthop. 2004. 18: 58-62
35. Mauffrey C, Randhawa K, Lewis C, Brewster M, Dabke H. Cauda equina syndrome: An anatomically driven review. Br J Hosp Med. 2008. 69: 344-7
36. Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. J Neurosurg. 1964. 21: 207-11
37. Muir JJ, Pingree MJ, Moeschler SM. Acute Cauda equina syndrome secondary to a lumbar synovial cyst. Pain Physician. 2012. 15: 435-40
38. Muthukumar T, Butt SH, Cassar-Pullicino VN, McCall IW. Cauda equina syndrome presentation of sacral insufficiency fractures. Skeletal Radiol. 2007. 36: 309-13
39. Poncelet A. The neurologic complications of Paget’s disease. J Bone Miner Res. 1999. 14: 66-9
40. Qureshi A, Sell P. Cauda equina syndrome treated by surgical decompression: The influence of timing on surgical outcome. Eur Spine J. 2007. 16: 2143-51
41. Radcliff KE, Kepler CK, Delasotta LA, Rihn JA, Harrop JS, Hilibrand AS. Current management review of thoracolumbar cord syndromes. Spine J. 2011. 11: 884-92
42. Raj D, Coleman N. Cauda equina syndrome secondary to lumbar disc herniation. Acta Orthop Belg. 2008. 74: 522-7
43. Ramdass S, Brennan M. A rare cause of Cauda equina syndrome by infiltration: Case of a sacral chordoma. J Am Geriatr Soc. 2015. 63: 1269-70
44. Sandhu GS, Ghufoor K, Gonzalez-Garcia J, Elexpuru-Camiruaga JA. Granulocytic sarcoma presenting as Cauda equina syndrome. Clin Neurol Neurosurg. 1998. 100: 205-8
45. Shapiro S. Medical realities of Cauda equina syndrome secondary to lumbar disc herniation. Spine (Phila Pa 1976). 2000. 25: 348-51
46. Siquara de Sousa AC, Capek S, Howe BM, Jentoft ME, Amrami KK, Spinner RJ. Magnetic resonance imaging evidence for perineural spread of endometriosis to the lumbosacral plexus: Report of 2 cases. Neurosurg Focus. 2015. 39: E15-
47. Spector LR, Madigan L, Rhyne A, Darden B, Kim D. Cauda equina syndrome. J Am Acad Orthop Surg. 2008. 16: 471-9
48. Stambough JL, Reid JH, Ross MA, Simeone FA, Booth RE. Briefly noted: Isolated intradural metastasis simulating lumbar disc disease. Spine (Phila Pa. 1976). 1991. 16: 581-3
49. Tait MJ, Chelvarajah R, Garvan N, Bavetta S. Spontaneous hemorrhage of a spinal ependymoma: A rare cause of acute Cauda equina syndrome: A case report. Spine (Phila Pa 1976). 2004. 29: E502-5
50. Tarulli AW. Disorders of the Cauda equina. Continuum (Minneap Minn). 2015. 21: 146-58
51. Todd NV. Cauda equina syndrome: The timing of surgery probably does influence outcome. Br J Neurosurg. 2005. 19: 301-6
52. Wells AJ, McDonald MJ, Sandler SJ, Vrodos NJ. Lumbosacral epidural lipomatosis causing rapid onset Cauda equina syndrome. J Clin Neurosci. 2014. 21: 1262-3
53. Wills JH, Wiesel S, Abram SE, Rupp FW. Synovial cysts and the lithotomy position causing Cauda equina syndrome. Reg Anesth Pain Med. 2004. 29: 234-6
Category: Neuropathology
Article Type: Case Report
Michael J. Benko, Aaron P. Danison, Eric A. Marvin, Brian F. Saway
Division of Neurosurgery, Virginia Tech Carilion School of Medicine Roanoke, Virginia, United States.
DOI:10.25259/SNI-152-2019
Copyright: © 2019 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
How to cite this article: Michael J. Benko, Aaron P. Danison, Eric A. Marvin, Brian F. Saway. Distal Cauda equina syndrome: A case report of lumbosacral disc pathology and review of literature. 10-May-2019;10:84
How to cite this URL: Michael J. Benko, Aaron P. Danison, Eric A. Marvin, Brian F. Saway. Distal Cauda equina syndrome: A case report of lumbosacral disc pathology and review of literature. 10-May-2019;10:84. Available from: http://surgicalneurologyint.com/surgicalint-articles/distal-cauda-equina-syndrome-a-case-report-of-lumbosacral-disc-pathology-and-review-of-literature/
Date of Submission
02-Dec-2018
Date of Acceptance
25-Feb-2019
Date of Publication
10-May-2019
Page views
127
Steiner SkyscraperElekta SkyscraperSteiner SkyscraperElekta Skyscraper
Abstract
Background: Cauda equina syndrome (CES) is an uncommon entity that presents acutely with all or some of the following symptoms; urinary incontinence from retention, fecal incontinence from loss of sphincter tone, saddle area hypoesthesia or anesthesia, and acute or progressive weakness in one or both lower extremities. The protean symptomatology is often mixed and is vulnerable to confounding comorbidities making the accurate and timely diagnosis of this syndrome uniquely challenging. Here, we present the case of a man who developed isolated sacral nerve dysfunction from CES in the midst of a diabetic crisis.
Case Description: A 53-year-old male with a long history of uncontrolled Type 2 diabetes presented with acute-onset urinary and fecal incontinence, scrotal anesthesia, and a 3-day history of lower back pain with intermittent bilateral leg pain. This patient displayed no objective changes in leg strength, sensation, or reflexes. In addition, the patient tested positive for cocaine and had a blood glucose level of 800 mg/dL which confounded his clinical picture. The patient underwent bilateral laminectomies from L4–S1 with the removal of a large sequestered disc fragment from the S1–S2 disc space within 8 h of presentation with reasonable recovery.
Conclusion: Highly variable presentations often confound the accurate and timely diagnosis of CES with severe implications on quality of life. Despite the limited functional recovery seen after surgical decompression, urgent or emergent intervention is paramount for treatment. Our patient’s presenting symptomatology and comorbidities highlight the need for practitioners to maintain a high index of suspicion in anyone with incontinence and back pain, regardless of distractors and even in the absence of other anticipated motor or sensory findings.
Keywords: Cauda, equina, herniation, neurosurgery, syndrome
INTRODUCTION
Cauda equina syndrome (CES) is an uncommon, but well-known surgical entity that is often seen in primary care clinics and emergency departments. It characteristically presents acutely with some or all of the following symptoms: urinary retention with or without overflow incontinence, fecal incontinence from loss of anal sphincter tone, saddle area hypoesthesia or anesthesia, and acute or progressive weakness in the muscles of one or both lower extremities.[ 17 , 27 , 28 ] Although the time frame for surgical decompression has not been clearly delineated, it is often considered a surgical emergency and the presence of a true CES is typically treated within 24–48 h if possible.[ 3 , 13 , 51 ] The incidence of CES is approximately 0.04% (1:2500) in all patients who present with low back pain and only 1–2% of patients with lumbar disc herniations which is the most common cause, though the etiology of CES is diverse and can be caused by tumor, trauma, and infections.[ 1 , 2 , 5 , 6 , 18 , 21 , 24 , 25 , 33 , 39 , 43 , 45 , 50 , 52 ] The documented incidence appears to be on the rise, however, and given the legal implications surrounding the diagnosis and misdiagnosis of this entity, the true clinical syndrome may be overreported in literature.
Despite CES being well established among spine surgeons, there are several variables that can often contradict the suspected diagnosis and lead to delays in treatment. These can include inconsistent portions of the reported history, the physical examination, and even lumbar imaging.[ 7 ] Given the physical and legal ramifications, ruling out CES becomes the clinical responsibility of the treating physician which can be both challenging and fraught with peril. We suggest that a high level of suspicion should be maintained when caring for any patient presenting with urinary or fecal incontinence and acute or chronic back pain, even in the absence of other associated motor or sensory findings. We now report an atypical presentation of distal CES caused by a large, sequestered, caudally migrated L5–S1 herniated disc fragment.
CASE REPORT
A 53-year-old male with a long history of uncontrolled Type 2 diabetes presented to the emergency department after recent cocaine ingestion with newly discovered scrotal numbness and several episodes of urinary and fecal incontinence. His finger-stick blood glucose was measured to be 800 mg/dL before consultation and his hemoglobin A1C was >18.5%. He endorsed mild-moderate low back pain for the previous 3 days with intermittent bilateral leg pain that was nondermatomal in distribution but denied any leg weakness or difficulty ambulating. His physical examination confirmed that his motor strength was full and his sensation to light touch was intact in all lower extremity muscle groups and tested dermatomes bilaterally. He also had normal patellar and Achilles deep tendon reflexes bilaterally. He did have perineal anesthesia to light touch and pinprick testing, as well as poor rectal tone. The patient had already received a Foley catheter before consultation without precatheter bladder volume assessment or documented initial urinary output.
He underwent an emergent lumbar magnetic resonance imaging which demonstrated mild-moderate multilevel degenerative spondylosis in the setting of congenital canal stenosis with significant lateral recess stenosis at L4–5 and a centrally herniated nucleus pulposus (HNP) at L5–S1 [ Figure 1 ]. After discussing the risks and benefits of surgical decompression, the patient elected to proceed and was brought to the operating room for surgery within 8 h. Bilateral posterior laminectomies were performed at L4 and L5. The patient was noted to have severe stenosis caudal to the S1 lamina intraoperatively, so this was also removed bilaterally and revealed a large sequestered herniated disc fragment [ Figure 2 ] posterior to the S1–S2 disc space [ Figure 3 ] causing severe sacral nerve compression. Although the fragment was not sent for microscopic evaluation, it was of typical appearance, texture, and size of an HNP, and there was a clear annular defect at L5–S1 consistent with its presumed origin. The patient did well after surgery with regard to his back pain, and his perineal sensation and bowel continence improved significantly during his hospitalization. He was discharged home with a Foley catheter and outpatient urology follow-up on postoperative day 5.
Figure 1
Lumbar magnetic resonance imaging demonstrating mild- moderate multilevel degenerative disease in the setting of congenital canal stenosis with significant lateral recess stenosis at L4–5 and a herniated nucleus pulposus at L5–S1.
Figure 2
Sequestered herniated disc fragment.
Figure 3
Radiograph demonstrating herniated disc fragment posterior to the S1–S2 disc space.
DISCUSSION
First described by Mixter and Barr, in 1934, the most common etiology of C. equina compression is an HNP.[ 36 ] Symptoms are a direct result of nerve root dysfunction and most authors would agree that micturition impairment should be present to diagnose CES.[ 13 , 30 , 41 , 47 ] Symptoms that support the diagnosis of CES include decreased anal sphincter tone, reduced sensation in the sacral dermatomes (saddle area), and sexual dysfunction.[ 3 , 5 , 28 , 35 , 42 ] It should be noted that symptoms of sciatica (neurological deficits in one or both lower extremities including changes in reflexes, motor function, or sensation) are more common[ 27 , 28 ] at the time of CES presentation and the prevalence of it has been quoted to be 97%, while micturition dysfunction was seen in 92%, saddle anesthesia in 93%, and defecation dysfunction found in 74% of patients.[ 28 ] However, many studies have identified discrepancies between physical examination findings, imaging findings, and accurate diagnosis highlighting the protean presentation and need for vigilance.[ 8 , 16 , 20 , 40 , 45 ]
It is estimated that fewer than 1 in 2000 of patients who present with acute severe lower back pain will be diagnosed with CES[ 49 ] and up to 19–41% of patients who are referred for CES evaluation have essentially negative imaging studies.[ 7 , 8 ] In fact, patients presenting to the emergency department with isolated low back pain rarely require urgent or emergent neurosurgical intervention,[ 11 , 14 , 31 ] yet there is a myriad of CES causes that can present with subacute or vague pain and symptomatology. Some well-documented etiologies include lesions such as intracanalicular hemorrhages,[ 12 , 25 ] tumors,[ 26 , 44 , 48 ] cysts,[ 37 , 53 ] lipomatosis,[ 52 ] infection,[ 1 ] and even perineural spread of endometriosis.[ 46 ] Moreover, inflammatory and degenerative conditions that lead to stenosis such as ankylosing spondylitis,[ 29 ] Paget’s disease,[ 39 ] osteoporotic stress fracture,[ 38 ] and hypertrophic degenerative stenosis[ 24 ] have also been well recognized in literature. While most pathologies have distinct imaging characteristics, there are various neoplastic causes of CES that has masqueraded as simple herniated discs including Ewing sarcoma,[ 22 ] osteosarcoma,[ 9 ] and even intradural metastatic cancer.[ 48 ] The vast etiological umbrella that underlies CES warrants a thorough workup on a case-by-case basis and should any doubt exist regarding intraoperative findings during the requisite surgical intervention, we would advocate that a specimen be provided for pathological analysis to confirm the etiology.
Despite CES being considered a surgical emergency with decompression often occurring in an expedient fashion, the postoperative resolution of deficits is historically quite poor.[ 19 ]
A meta-analysis by Korse et al., in 2017, found that at postoperative follow-up, the prevalence of residual sciatica was 48%, micturition dysfunction was 48%, altered saddle sensation was 57%, defecation dysfunction was 42%, and sexual dysfunction was 53%.[ 28 ] This emphasizes the point that assessing patients with this constellation of symptoms must be taken seriously as the impact on long-term quality of life is quite profound even with proper treatment. Moreover, comorbidities such as hyperglycemic neuropathy in a diabetic may have elements of these symptoms at baseline and can act as a distractor in cases of suspected CES as in our patient.[ 15 ] Indeed, CES diagnosis often becomes a mounting medicolegal issue with at least one study documenting that in 96% of cases of CES brought to a malpractice insurance organization, 65% progressed to claims by the affected patients, and 48% of these cases resulted in payment of compensation for damages.[ 34 ] It must be noted that rare cases of CES resulting from iatrogenic causes such as retained surgical cottonoids,[ 4 ] epidural steroid injections,[ 10 ] and even a pathological engorgement of the epidural venous plexus after elective lumbar microdiscectomy[ 32 ] have been reported in literature with additional consequences. Furthermore, studies have shown that a delay in treatment typically results in adverse legal decision highlighting the requisite expedited intervention.[ 19 , 23 ]
CONCLUSION
As in our patient with clinical distractors and undocumented bladder dysfunction before consultation, the diagnosis of CES often becomes difficult to specify and confirm, so we ultimately advocate for physicians to maintain a high index of suspicion for CES in any patient presenting with concern for sacral nerve dysfunction.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patients understand that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agarwal N, Shah J, Hansberry DR, Mammis A, Sharer LR, Goldstein IM. Presentation of Cauda equina syndrome due to an intradural extramedullary abscess: A case report. Spine J. 2014. 14: e1-6
2. Ahn H, Jhaveri S, Yee A, Finkelstein J, Ahn H, Jhaveri S. Lumbar vertebral hemangioma causing Cauda equina syndrome: A case report. Spine. 2005. 30: E662-4
3. Ahn UM, Ahn NU, Buchowski JM, Garrett ES, Sieber AN, Kostuik JP. Cauda equina syndrome secondary to lumbar disc herniation: A meta-analysis of surgical outcomes. Spine (Phila Pa. 1976). 2000. 25: 1515-22
4. Akhaddar A, Baallal H, Elktaibi A. Abscess due to textiloma (gossypiboma: Retained surgical cottonoid). Surg Neurol Int. 2018. 9: 70-
5. Andersen JT, Bradley WE. Neurogenic bladder dysfunction in protruded lumbar disk and after laminectomy. Urology. 1976. 8: 94-6
6. Bal S, Kurtulmuş Ş, Koçyiğit H, Gürgan A. A case with Cauda equina syndrome due to bacterial meningitis of anterior sacral meningocele. Spine (Phila Pa. 1976). 2004. 29: E298-9
7. Balasubramanian K, Kalsi P, Greenough CG, Seetharam MP. Reliability of clinical assessment in diagnosing Cauda equina syndrome. Br J Neurosurg. 2010. 24: 383-6
8. Bell DA, Collie D, Statham PF. Cauda equina syndrome-what is the correlation between clinical assessment and MRI scanning?. Br J Neurosurg. 2007. 21: 201-3
9. BeşkonaklI E, Çayli SR, Okay Ö, Bostanci U, Ergün R. Pelvic osteosarcomas causing lumbosacral nerve root compression presenting as herniated intervertebral disc: Report of two cases. Neurosurg Rev. 2000. 23: 165-7
10. Bilir A, Gulec S. Cauda equina syndrome after epidural steroid injection: A case report. J Manipulative Physiol Ther. 2006. 29: 492.e1-e3
11. Carragee EJ, Hannibal M. Diagnostic evaluation of low back pain. Orthop Clin North Am. 2004. 35: 7-16
12. Cordan T, Bekar A, Yaman O, Tolunay Ş. Spinal subarachnoid hemorrhage attributable to schwannoma of the Cauda equina. Surg Neurol. 1999. 51: 373-5
13. DeLong WB, Polissar N, Neradilek B. Timing of surgery in Cauda equina syndrome with urinary retention: Meta-analysis of observational studies. J Neurosurg Spine. 2008. 8: 305-20
14. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain?. J Am Med Assoc. 1992. 268: 760-5
15. Dolman CL. Morbid anatomy of diabetic neuropathy. JAMA. 1963. 183: 225-
16. Domen PM, Hofman PA, Van Santbrink H, Weber WE. Predictive value of clinical characteristics in patients with suspected Cauda equina syndrome. Eur J Neurol. 2009. 16: 416-9
17. Fraser S, Roberts L, Murphy E. Cauda equina syndrome: A literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009. 90: 1964-8
18. Gitelman A, Hishmeh S, Morelli BN, Joseph SA, Casden A, Kuflik P. Cauda equina syndrome: A comprehensive review. Am J Orthop (Belle Mead NJ). 2008. 37: 556-62
19. Gleave JR, MacFarlane R. Cauda equina syndrome: What is the relationship between timing of surgery and outcome?. Br J Neurosurg. 2002. 16: 325-8
20. Gooding BW, Higgins MA, Calthorpe DA. Does rectal examination have any value in the clinical diagnosis of Cauda equina syndrome?. Br J Neurosurg. 2013. 27: 156-9
21. Hamdan A, Strachan RD, Nath F, Coulter IC. Counting the cost of negligence in neurosurgery: Lessons to be learned from 10 years of claims in the NHS. Br J Neurosurg. 2015. 29: 169-77
22. Hsieh CT, Chiang YH, Tsai WC, Sheu LF, Liu MY. Primary spinal epidural ewing sarcoma: A case report and review of the literature. Turk J Pediatr. 2008. 50: 282-6
23. Hussain SA, Gullan RW, Chitnavis BP. Cauda equina syndrome: Outcome and implications for management. Br J Neurosurg. 2003. 17: 164-7
24. Joffe R, Appleby A, Arjona V. Intermittent ischaemia of the Cauda equina due to stenosis of the lumbar canal. J Neurol Neurosurg Psychiatry. 1966. 29: 315-8
25. Kebaish KM, Awad JN. Spinal epidural hematoma causing acute Cauda equina syndrome. Neurosurg Focus. 2004. 16: e1-
26. Kim DY, Lee JK, Moon SJ, Kim SC, Kim CS. Intradural spinal metastasis to the Cauda equina in renal cell carcinoma: A case report and review of the literature. Spine (Phila Pa. 1976). 2009. 34: E892-5
27. Korse NS, Jacobs WC, Elzevier HW, Vleggeert-Lankamp CL. Complaints of micturition, defecation and sexual function in Cauda equina syndrome due to lumbar disk herniation: A systematic review. Eur Spine J. 2013. 22: 1019-29
28. Korse NS, Pijpers JA, van Zwet E, Elzevier HW, Vleggeert-Lankamp CL. Cauda equina syndrome: Presentation, outcome, and predictors with focus on micturition, defecation, and sexual dysfunction. Eur Spine J. 2017. 26: 894-904
29. Lo C, Nair KP, Romanowski CA, Fawthorpe FW, Lindert RB. Horse’s tail in bamboo spine: The ‘Cauda equina syndrome in ankylosing spondylitis. Pract Neurol. 2014. 14: 418-21
30. Ma B, Wu H, Jia L, Yuan W, Shi G, Shi J. Cauda equina syndrome: A review of clinical progress. Chin Med J (Engl). 2009. 122: 1214-22
31. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017. 389: 736-47
32. Maki Y, Takayama M, Hayashi H, Yokoyama Y, Agawa Y. Cauda equina syndrome due to dural sac shift with engorgement of the epidural venous plexus: Rare complication after lumbar microdiscectomy. World Neurosurg. 2017. 104: e15-8
33. Maloney P. Handbook of neurosurgery. Yale J Biol Med. 2008. 81: 210-1
34. Markham DE. Cauda equina syndrome: Diagnosis, delay and litigation risk. Curr Orthop. 2004. 18: 58-62
35. Mauffrey C, Randhawa K, Lewis C, Brewster M, Dabke H. Cauda equina syndrome: An anatomically driven review. Br J Hosp Med. 2008. 69: 344-7
36. Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. J Neurosurg. 1964. 21: 207-11
37. Muir JJ, Pingree MJ, Moeschler SM. Acute Cauda equina syndrome secondary to a lumbar synovial cyst. Pain Physician. 2012. 15: 435-40
38. Muthukumar T, Butt SH, Cassar-Pullicino VN, McCall IW. Cauda equina syndrome presentation of sacral insufficiency fractures. Skeletal Radiol. 2007. 36: 309-13
39. Poncelet A. The neurologic complications of Paget’s disease. J Bone Miner Res. 1999. 14: 66-9
40. Qureshi A, Sell P. Cauda equina syndrome treated by surgical decompression: The influence of timing on surgical outcome. Eur Spine J. 2007. 16: 2143-51
41. Radcliff KE, Kepler CK, Delasotta LA, Rihn JA, Harrop JS, Hilibrand AS. Current management review of thoracolumbar cord syndromes. Spine J. 2011. 11: 884-92
42. Raj D, Coleman N. Cauda equina syndrome secondary to lumbar disc herniation. Acta Orthop Belg. 2008. 74: 522-7
43. Ramdass S, Brennan M. A rare cause of Cauda equina syndrome by infiltration: Case of a sacral chordoma. J Am Geriatr Soc. 2015. 63: 1269-70
44. Sandhu GS, Ghufoor K, Gonzalez-Garcia J, Elexpuru-Camiruaga JA. Granulocytic sarcoma presenting as Cauda equina syndrome. Clin Neurol Neurosurg. 1998. 100: 205-8
45. Shapiro S. Medical realities of Cauda equina syndrome secondary to lumbar disc herniation. Spine (Phila Pa 1976). 2000. 25: 348-51
46. Siquara de Sousa AC, Capek S, Howe BM, Jentoft ME, Amrami KK, Spinner RJ. Magnetic resonance imaging evidence for perineural spread of endometriosis to the lumbosacral plexus: Report of 2 cases. Neurosurg Focus. 2015. 39: E15-
47. Spector LR, Madigan L, Rhyne A, Darden B, Kim D. Cauda equina syndrome. J Am Acad Orthop Surg. 2008. 16: 471-9
48. Stambough JL, Reid JH, Ross MA, Simeone FA, Booth RE. Briefly noted: Isolated intradural metastasis simulating lumbar disc disease. Spine (Phila Pa. 1976). 1991. 16: 581-3
49. Tait MJ, Chelvarajah R, Garvan N, Bavetta S. Spontaneous hemorrhage of a spinal ependymoma: A rare cause of acute Cauda equina syndrome: A case report. Spine (Phila Pa 1976). 2004. 29: E502-5
50. Tarulli AW. Disorders of the Cauda equina. Continuum (Minneap Minn). 2015. 21: 146-58
51. Todd NV. Cauda equina syndrome: The timing of surgery probably does influence outcome. Br J Neurosurg. 2005. 19: 301-6
52. Wells AJ, McDonald MJ, Sandler SJ, Vrodos NJ. Lumbosacral epidural lipomatosis causing rapid onset Cauda equina syndrome. J Clin Neurosci. 2014. 21: 1262-3
53. Wills JH, Wiesel S, Abram SE, Rupp FW. Synovial cysts and the lithotomy position causing Cauda equina syndrome. Reg Anesth Pain Med. 2004. 29: 234-6
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου