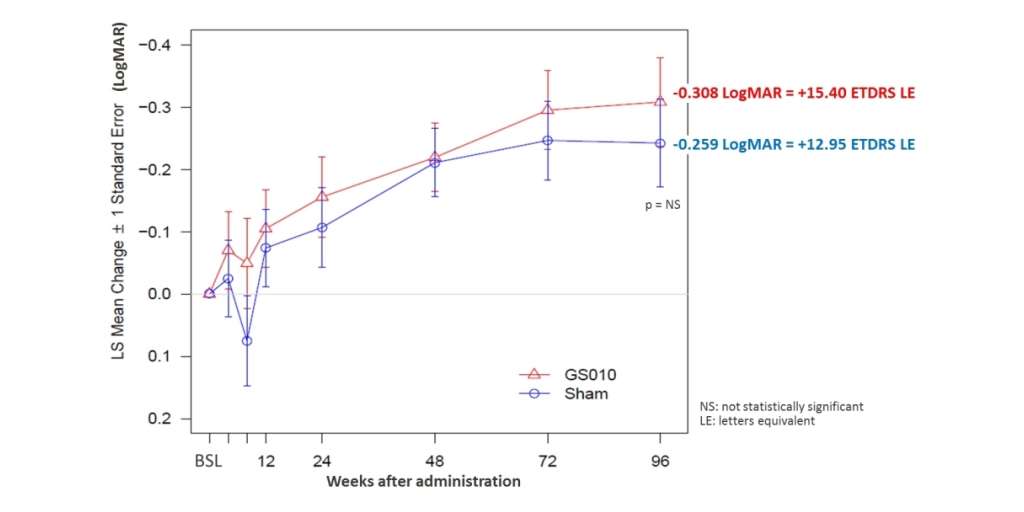
PARIS--(BUSINESS WIRE)--Regulatory News: GenSight Biologics (Paris:SIGHT) (Euronext: SIGHT, ISIN: FR0013183985, PEA-PME eligible), a biopharma company focused on discovering and developing innovative gene therapies for retinal neurodegenerative diseases and central nervous system disorders, today reported a first set of results from Week 96 of the REVERSE Phase III clinical trial. The trial evaluated the safety and efficacy of a single intravitreal injection of GS010 (rAAV2/2-ND4) in 37 subject
Read Full Article
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου