Dental remineralization via poly(amido amine) and restorative materials containing calcium phosphate nanoparticles
International Journal of Oral Science 11, Article number: 15 (2019)
Abstract
Tooth decay is prevalent, and secondary caries causes restoration failures, both of which are related to demineralization. There is an urgent need to develop new therapeutic materials with remineralization functions. This article represents the first review on the cutting edge research of poly(amido amine) (PAMAM) in combination with nanoparticles of amorphous calcium phosphate (NACP). PAMAM was excellent nucleation template, and could absorb calcium (Ca) and phosphate (P) ions via its functional groups to activate remineralization. NACP composite and adhesive showed acid-neutralization and Ca and P ion release capabilities. PAMAM+NACP together showed synergistic effects and produced triple benefits: excellent nucleation templates, superior acid-neutralization, and ions release. Therefore, the PAMAM+NACP strategy possessed much greater remineralization capacity than using PAMAM or NACP alone. PAMAM+NACP achieved dentin remineralization even in an acidic solution without any initial Ca and P ions. Besides, the long-term remineralization capability of PAMAM+NACP was established. After prolonged fluid challenge, the immersed PAMAM with the recharged NACP still induced effective dentin mineral regeneration. Furthermore, the hardness of pre-demineralized dentin was increased back to that of healthy dentin, indicating a complete remineralization. Therefore, the novel PAMAM+NACP approach is promising to provide long-term therapeutic effects including tooth remineralization, hardness increase, and caries-inhibition capabilities.
Introduction
Tooth decay is the most common oral disease, which is mainly due to the demineralization of dental hard tissues1,2,3,4. In the oral environment, there is a physiological equilibrium between the demineralization and remineralization5. However, when the acids from cariogenic bacteria increase, the balance tilts toward to demineralization and net mineral loss, eventually leading to caries3,6. Therefore, it would be highly beneficial to develop a new generation of bioactive and therapeutic dental materials that have functionalities to suppress demineralization and promote remineralization.
Hydroxyapatite (HA) is the main ingredient of tooth minerals7. When in contact with saliva, the following reaction occurs8:
Hence, promoting tooth remineralization requires: (1) increasing the calcium (Ca) and phosphate (P) ion concentrations; and (2) raising the local pH where remineralization is needed.
The demineralized dentin is mainly composed of collagen fibrils, which only have a weak nucleation template capability9,10. Therefore, it is important to supply nucleation template materials for the demineralized dentin to attract Ca and P ions, thereby activating and accelerating the remineralization process. Previously published meritorious reviews on dental remineralization are available5,11,12,13,14,15. The present article represents the first review that focuses on remineralization via novel poly(amido amine) (PAMAM) dendrimers having great nucleation capability, together with a new generation of bioactive resins with acid-neutralization, as well as Ca and P ion recharge and re-release functions.
Using PAMAM as nucleation templates for dental remineralization
Nature is rich with examples of biomineralized hard tissues possessing outstanding mechanical properties and complex morphologies that emerge from their hierarchically arranged structures16. Dentin and enamel are two of those examples. Dental caries and erosion are common oral diseases. To promote the remineralization of dentin or enamel via coating remineralization materials on the tooth is a non-invasive therapeutic technique in clinical practice3. Dentin is composed of HA, organic matrix, and water17. Collagen fibrils are the main component of the demineralized dentin. Some studies showed that collagen fibrils did not have the ability to induce HA nucleation or growth18,19, while other studies indicated that collagen fibrils could initialize nucleation and apatite deposit through its carbonyl and carboxy groups20,21. However, the nucleation rate of collagen fibrils is far too slow without the use of nucleation template materials10. Non-collagenous proteins (NCPs), although composing of only about 3% of the organic components of dentin, play a vital role in the modulation of the biomineralization process22,23,24,25. NCPs are regarded as the nucleation templates within the collagen fibrils, which can control the hierarchical growth of HA26,27,28,29. In mature dentin, however, NCPs lose the ability to initialize remineralization14. Thus, the strategy of looking for analog materials that act in the role of NCPs has been studied. Mature enamel consists of ~96% of highly organized hierarchical prisms which are derived from nanorod-like HA crystals, and 4% of organic compounds. Extracellular matrix proteins, of which amelogenin is a well-known example, act as nucleation templates in the biomineralization process of enamel30,31,32. Once mature enamel is demineralized, it cannot regenerate because there are no ameloblasts to secrete amelogenin. Therefore, designing analog materials that play the role of extracellular matrix proteins to acquire the hierarchical structure of enamel is needed to repair the defective enamel.
Mimicking the functions of NCPs, phosphate-based template analogs of matrix proteins were synthesized by immobilizing amorphous calcium phosphate (ACP) to induce apatite nucleation and growth at intrafibrillar locations33,34,35,36. Polydopamine-induced effective biomimetic remineralization of demineralized dentin10. In addition, amelogenin, enamel matrix derivative, and gelatin were applied to initiate the growth of aligned apatite nanocrystals on enamel37,38,39. Furthermore, the disorder–order interplay of elastin-like recombinamers were exploited to enable highly controlled and hierarchical mineralization resembling enamel40.
PAMAM dendrimers were investigated as nucleation templating analogs for biomineralization41. PAMAM are highly branched polymers with internal cavities and a large number of reactive terminal groups41. Several different generations of PAMAM with different structures were synthesized42. The first and second generations of PAMAM are linear molecules, while the third and higher generations are sphere molecules with a larger number of functional groups41. PAMAM have diversified types of terminal groups, including carboxyl-terminated PAMAM (PAMAM–COOH), hydroxy-terminated PAMAM (PAMAM–OH), amine-terminated PAMAM (PAMAM–NH2), and phosphorylated PAMAM (PAMAM–PO3H2).
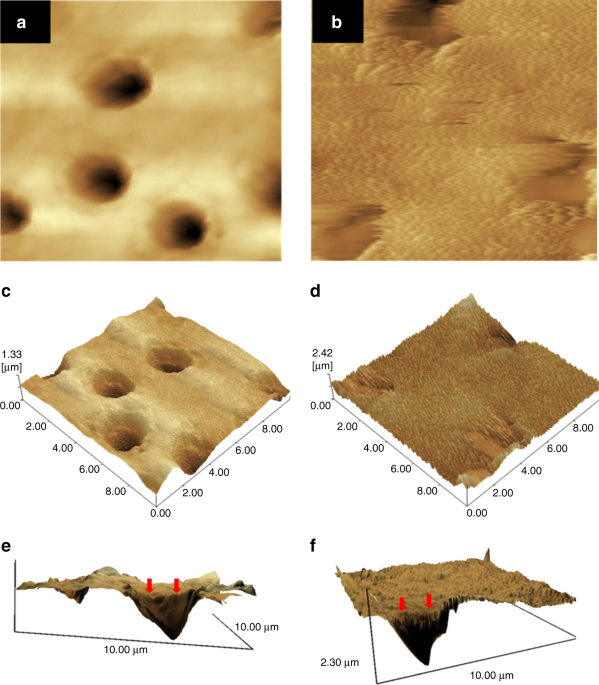
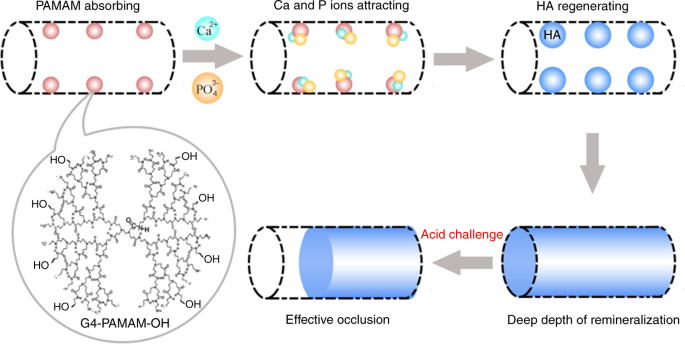
Studies showed that PAMAM–COOH acted as the organic nucleation template to induce biomimetic new-grown crystals on the demineralized enamel.43,44 PAMAM–COOH absorbed Ca and P ions within collagen fibrils to induce intrafibrillar remineralization. The mineralization process initiated by PAMAM–COOH was illustrated and characterized through both in vitro and in vivo experiments45. PAMAM–PO3H2 was absorbed on demineralized enamel surface tightly and produced an enamel prism-like structure, which was also proved in an animal model46. PAMAM–PO3H2 bound tightly to dentin collagen fibrils, and induced effective regeneration of demineralized dentin47,48. In addition, PAMAM–NH2 induced the regeneration of minerals on both collagen fibril surface and demineralized dentin surface (Fig. 1)49,50,51,52. Moreover, PAMAM–OH showed a moderate remineralization ability on occluding dentinal tubules53. Fig. 2 shows a schematic of effective dentinal tubule occlusion induced by PAMAM–OH53. Furthermore, a study concluded that PAMAM–NH2 and PAMAM–COOH were more effective than PAMAM–OH on dentin remineralization, because –NH2and –COOH groups had stronger abitlity than –OH group to grab Ca and P ions54.
AFM images of the demineralized dentin surfaces without treatment (a, c, e) and with the treatment of PAMAM-NH2 (b, d, f) after being immersed in artificial saliva for 4 weeks (image area: 10 μm × 10 μm). a and b are the topographical images. c and d are the three-dimensional images. e and f are reconstructed from a and b by the software SPM-9700, and a horizontal plane of the remineralization surface and longitudinal profile of dentinal tubule were observed. Control showed no mineral regeneration. In contrast, PAMAM–NH2 induced large amounts of needle-like minerals in dentin. (Adapted from Liang et al.50 with permission.)
First, PAMAM–OH macromolecules bound to collagen fibrils within the tubules through size-exclusion features of collagen fibrils and electrostatic interactions. Then the templates served as a nucleation site to attract calcium ions through calcium complexation by both amide groups and hydroxy groups. Then the calcium ions attracted phosphate ions to form HA. The stable intratubular minerals could resist the acid attack, which ensured that the dentinal tubule occlusion was effective under acidic oral environment. (Adapted from Liang et al.53 with permission.)
The remineralization mechanism induced by PAMAM was still not clear. Some researchers speculated one possible mechanism: PAMAM–NH2macromolecules bind to dentin collagen fibrils via size-exclusion features and electrostatic interactions of collagen50. According to size-exclusion feature theories, molecules with a molecular weight between 6 and 40 kDa can be retained within the dentin collagen fibrils55. On the other hand, the positively or negatively charged groups of PAMAM–NH2macromolecules help themselves to bind to the charged sites on collagen fibrils via electrostatic interactions50. The immobilized PAMAM–NH2 macromolecules then play the role of nucleation templates, mimic the functions of organic matrix proteins, thus initializing and accelerating the remineralization process. To date, besides the mechanism of using PAMAM as nucleation templates for remineralization, there have been no reports on other possible remineralization mechanisms via PAMAM. Further study is needed to investigate other remineralization mechanisms via PAMAM.
Dental composites and adhesives containing calcium phosphate nanoparticles with remineralization capability
Resin composites are popular due to their excellent esthetics, direct-filling capability and enhanced performance56,57,58,59,60,61. However, the demineralization at the restoration margins due to biofilm acids often leads to secondary caries, which is the main reason for restoration failures62,63. To address this problem, remineralization ingredients were incorporated into composites64,65,66,67,68,69,70. Since fluoride is widely used for dental remineralization, fluoride-releasing features were incorporated in the development of bioactive dental composites with remineralization capabilities. Fluorine (F) ions can be incorporated into tooth minerals, leading to fluoroapatite or F-enriched HA, both having a reduced solubility. Hence, F-releasing materials could help inhibit demineralization and facilitate remineralization. Recently, calcium fluoride (CaF2) nanoparticles were developed via a spray-drying system71. The CaF2 nanocomposite showed high F ion release and good mechanical properties, which may promote remineralization and reduce secondary caries72,73. However, the opacity of the CaF2nanocomposite will hinder uses where a high level of esthetics is desired, especially when the nano-CaF2 filler level is relatively high72,73.
Another important approach was to develop calcium phosphate (CaP) resin composites68,74,75,76,77. Traditional CaP composites, containing CaP fillers with particle sizes of ~1–55 μm, released high levels of Ca and P ions, and remineralized tooth lesions in vitro74,75,76. However, their mechanical properties were too weak for bulk filling applications. Hence, one study developed whisker-reinforced CaP composite with improved mechanical properties78. In another study, a barium-glass filler was incorporated into a nanocomposite containing ACP, which yielded an increase in mechanical properties, with no negative influence on ions release79. However, the mechanical properties of these traditional CaP composites were still weak, with flexural strength being only about half that of the unfilled resin74,75,76.
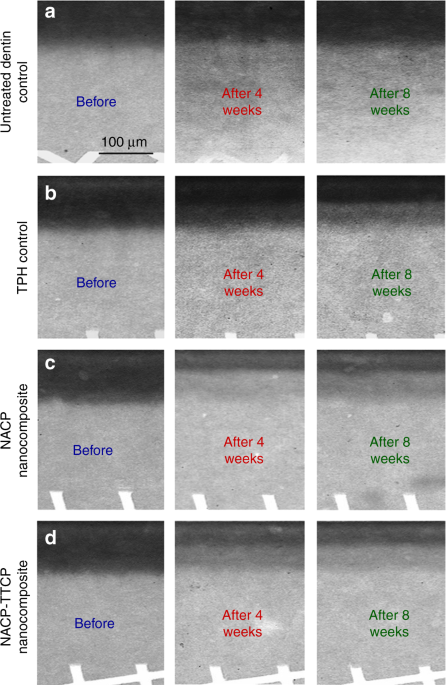
More recently, nanoparticles of ACP (NACP) with particle sizes of about 100 nm were synthesized80,81,82,83,84,85,86. The NACP composite released supersaturating levels of Ca and P ions, while possessing flexural strength and elastic modules two-fold those of traditional CaP composites80. The NACP composite was “smart”, which greatly increased its ions release in an acidic condition, and rapidly neutralized acids, raising a cariogenic pH of 4 to a safe pH of above 5.587. Indeed, NACP composite was shown to promote tooth remineralization88,89,90. In an in vitro study, NACP composite remineralized artificial enamel lesions in a cyclic demineralization/remineralization regimen, inducing a remineralization that was four-fold that of a commercial F-releasing composite88. In another study, NACP composite facilitated dentin remineralization, achieving a remineralization much higher than that of a commercial nanocomposite (Fig. 3)89. In a human in situ model, 25 volunteers wore palatal devices containing enamel slabs with cavities restored with NACP or control composite. The NACP composite showed great caries-inhibition under biofilm acids in the oral environment, with enamel mineral loss that was only 1/3 that of a control composite90.
The left column, before, refers to the initial dentin demineralization created in the acidic solution. The middle column is after 4 weeks of the cyclic demineralization/remineralization regimen. The right column is after 8 weeks of the cyclic demineralization/remineralization regimen. NACP composite achieved a much greater remineralization than a commercial control composite. (Adapted from Weir et al.89 with permission.)
Besides composites, NACP were also incorporated into adhesives91,92,93. Due to the small size of NACP, the nanoparticles flowed with the adhesive into dentinal tubules to form bioactive resin tags91,92,93. The NACP adhesive infiltrated into the demineralized dentin collagen matrix to form a hybrid layer (HL)94,95,96,97. Mineral crystals can protect the HL from external risk factors. Therefore, promoting the remineralization of HL is a promising strategy to prolong the longevity of the resin–dentin bonds98,99,100. The NACP adhesive inhibited dentin demineralization by neutralizing the acids, and promoted the dentin remineralization by releasing large amounts of Ca and P ions101. Therefore, the NACP adhesive is promising to help prolong the resin–dentin-bonded interface, which warrants further investigation.
Remineralization via the combination of PAMAM with NACP
As we stated above, inducing dentin remineralization requires: (1) providing nucleation templates; (2) increasing the Ca and P ion concentrations; and (3) raising the local pH. PAMAM is an excellent nucleation template and can activate the remineralization process by absorbing Ca and P ions47,50,51,53. However, PAMAM cannot neutralize acids nor release Ca and P ions. In contrast, NACP can rapidly raise the local pH via neutralizing acids, and release large amounts of Ca and P ions87,88,89. Therefore, it would be advantageous to combine NACP with PAMAM to achieve triple benefits: excellent nucleation template, high level of Ca and P ions release, and strong acid-neutralization capacity. This unique synergistic effect between PAMAM and NACP is a major advantage of the PAMAM+NACP approach, when compared with other remineralization materials.
In a recent study102, the combination of PAMAM and NACP composite was used to induce dentin remineralization. A PAMAM solution was prepared by dissolving PAMAM powder (50 mg) in deionized water (50 mL) to achieve a concentration of 1 mg•mL–1. NACP composite was fabricated by incorporating 30% NACP and 40% glass particles into a resin. Demineralized dentin samples were: (1) treated with deionized water, (2) coated with PAMAM solution, (3) placed in contact with NACP composite, and (4) coated with PAMAM and then placed with NACP composite. Each day, the four groups were immersed in 1 mL of pH 7 artificial saliva for 23 h, and then in 1 mL of pH 4 lactic acid solution for 1 h at 37 °C. This pH cycling was repeated for 21 days. PAMAM and NACP composite each alone facilitated moderate remineralization. In contrast, the PAMAM+NACP combination induced much greater remineralization, with large amounts of needle-like mineral crystals in dentin surfaces and in dentinal tubules. In addition, the PAMAM+NACP combination increased the hardness of the pre-demineralized dentin back to that of healthy dentin, indicating a complete remineralization of the pre-demineralized dentin.
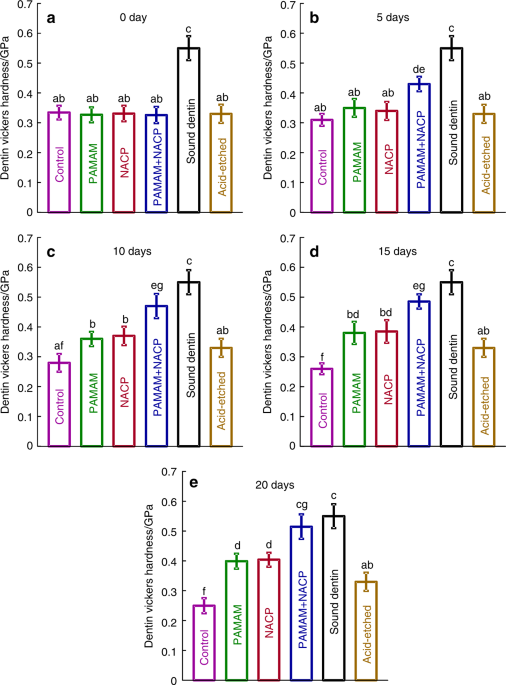
Besides composite, PAMAM+NACP bonding agent was also investigated for dentin remineralization101. A PAMAM solution with a concentration of 10 mg•mL–1 was prepared, and a bonding agent containing 40% NACP was fabricated. Four groups were examined: (1) control dentin, (2) dentin coated with PAMAM, (3) dentin in contact with NACP bonding agent, and (4) dentin with PAMAM+NACP bonding agent. The four groups were treated with a cyclic artificial saliva/lactic acid regimen for 10 days. PAMAM induced mild remineralization via its nucleation template function. NACP bonding agent neutralized acids to inhibit demineralization in lactic acid, and released Ca and P ions in artificial saliva, producing a moderate remineralization. In contrast, the PAMAM+NACP bonding agent combination induced the greatest dentin remineralization, with the most mineral regeneration and the greatest increase in dentin hardness (Fig. 4)101. Therefore, the novel PAMAM+NACP combination strategy showed excellent remineralization capability, and is promising for a wide range of dental applications to prolong the resin–dentin bonds, protect dental hard tissues, and inhibit secondary caries. Further studies using human in situ models are needed to investigate the efficacy and mechanisms of PAMAM+NACP in vivo.
Hardness of healthy dentin and acid-etched dentin were also measured as comparative controls. The NACP filler level was 40%. PAMAM and NACP each alone mildly increased the hardness of demineralized dentin. PAMAM+NACP achieved the most dentin remineralization, yielding the greatest increase in dentin hardness, which approached that of healthy dentin (P > 0.1). In each plot, dissimilar letters indicate significantly different values (P < 0.05). (Adapted from Liang et al.101 with permission.)
Remineralization in an extreme acid challenge environment via PAMAM+NACP
Saliva plays an important role for healthy individuals to inhibit caries103,104,105,106. With a constant flow, saliva helps remove bacteria via swallowing. Saliva can also neutralize biofilm acids by its strong buffer capability103,104,105,106. In addition, the high concentrations of Ca and P ions in saliva help promote remineralization. However, many factors can cause saliva reduction, such as Sjögren’s syndrome, nutrition deficiency, salivary dysfunction, certain medicines and systemic diseases, etc.103,104,105,106. Indeed, about one-third of the population reports dry mouth104.
For individuals with saliva reduction, the saliva flow could drop to about 15–33% of its normal level107. This means much less saliva to penetrate into bacteria biofilms to neutralize acids and provide Ca and P ions. This leads to an acidic and Ca and P ion-deficient microenvironment around the tooth, thus increasing the caries risk. Saliva reduction occurs often in seniors. Indeed, in the USA, root caries increased with aging, from 7% in young generation, to 56% among people of ≥75 years of age108. Furthermore, extreme saliva reduction often occurs in patients with head and neck cancers taking radiation therapy, and the extreme acidic and ion-deficient oral environment often causes rampant radiation caries109,110,111. Traditional caries-inhibition strategies are ineffective for patients with severe saliva reduction. For example, in an in vivo study, casein phosphopeptide ACP (CPP-ACP) failed to prevent radiation caries112. Therefore, there is a great need for new remineralization strategies that are effective in an acidic and Ca and P ion-deficient environment.
For the first time, in a pH 4 lactic acid solution without any initial Ca and P ions, the combination of PAMAM and NACP method successfully promoted dentin remineralization113,114. Demineralized dentin samples with (1) PAMAM, (2) NACP, and (3) PAMAM+NACP were immersed in pH 4 lactic acid solution for 24 h, and the solution was replaced by fresh pH 4 lactic acid solution each day. The 24 h of lactic acid immersion each day was used to simulate the most severely challenging oral condition for patients with the most severe saliva reduction. This was repeated for 21 days or 14 days, for NACP composite and NACP adhesive, respectively. The results showed that PAMAM did not cause any positive effects on protecting dentin structure in the lactic acid. This was because PAMAM could not fulfill its nucleation template function in an environment without any Ca and P ions. NACP prevented further dentin demineralization by neutralizing acids and releasing Ca and P ions. Remarkably, the PAMAM+NACP method achieved dentin remineralization even in the lactic acid, due to the synergy between PAMAM and NACP: NACP rapidly raised the pH of lactic acid to above 6 and released high levels of Ca and P ions, turning the lactic acid to a remineralization solution (Fig. 5)114. Then, PAMAM employed the Ca and P ions to facilitate remineralization, thus regenerating the minerals in the demineralized dentin (Fig. 6)113. The PAMAM+NACP composite increased the hardness of the demineralized dentin from 0.32 to 0.43 GPa at 7 days, to 0.49 GPa at 14 days, and to 0.53 GPa at 21 days, which approached that of healthy dentin113. In addition, the combination of PAMAM and NACP adhesive increased the dentin hardness from 0.31 to 0.56 GPa at 28 days, which was close to that of healthy dentin114. Therefore, the PAMAM+NACP synergy is promising to protect the teeth for seniors with gingiva recession and root caries, individuals with dry mouth, and especially those with severe saliva reduction and radiation caries. Further study should investigate the PAMAM+NACP remineralization in situ for the individuals with reduced saliva in the oral environment.
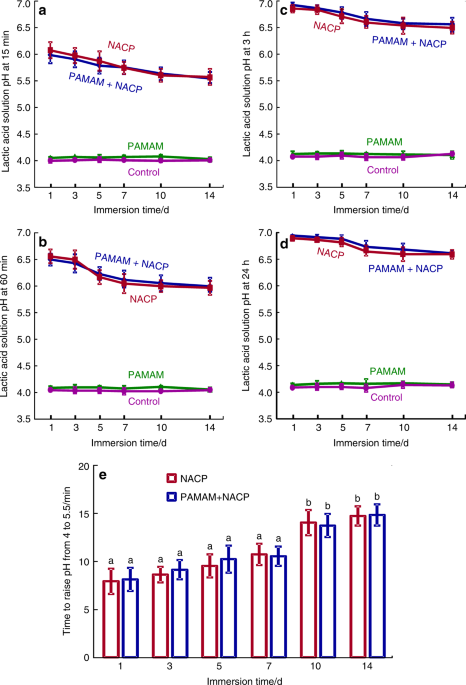
The lactic acid pH was measured after the sample was immersed for: (a) 15 min, (b) 60 min, (c) 3 h, and (d) 24 h (mean ± SD; n = 6). This was repeated for 14 days. (e) The time it took to raise the pH from 4 to 5.5. NACP and PAMAM+NACP neutralized the acid and increased the pH. The acid neutralization ability of NACP adhesive decreased over time due to the use of a fresh acid solution each day. PAMAM and control groups showed no acid neutralization, with pH staying near 4. (Adapted from Liang et al.114 with permission.)
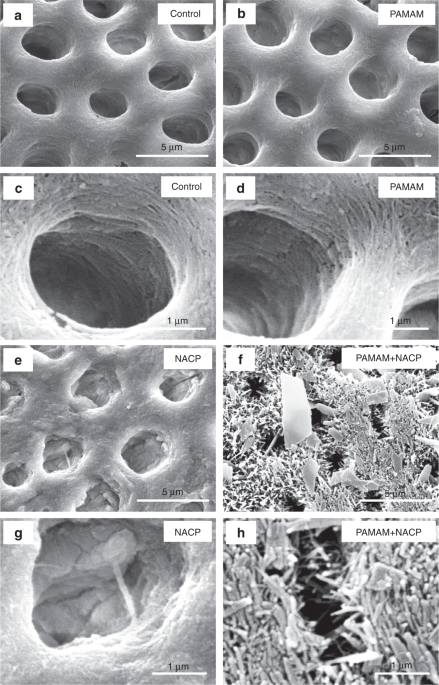
Representative SEM images of demineralized dentin perpendicular to tubule axis after 21 days lactic acid immersion: (a, c) control, (b, d) PAMAM, (e, g) NACP, and (f, h) PAMAM+NACP. Exposed collagen fibrils were observed in (c and d). Images (e–h) showed regenerated minerals in demineralized dentin. For PAMAM+NACP group in image (h), numerous needle-like minerals precipitated in dentin and occluded the tubules. (Adapted from Liang et al. 113with permission.)
PAMAM+NACP induced long-term dentin remineralization
Although the PAMAM+NACP method exhibited excellent remineralization capability, the ions release and acid-neutralization capabilities of NACP decreased with time101,102,113,114. In addition, with the fluids flow in the oral environment, PAMAM may be detached from the dental hard tissues with time, thus removing the nucleation templates. The process of secondary caries usually takes more than a year to form115,116. Therefore, the remineralization capability of PAMAM+NACP needs to be long-term.
Rechargeable NACP composite was developed using a resin of ethoxylated bisphenol A dimethacrylate (EBPADMA) and pyromellitic glycerol dimethacrylate (PMGDM), which was referred to as EBPM117,118. This EBPM+NACP composite could be repeatedly recharged with Ca and P ions, and then re-released these ions117,118. The mechanisms of the recharge process likely involves: (1) the Ca ion chelating capacity of PMGDM, and (2) the Ca and P ion space-occupying effect119. In addition, a rechargeable NACP adhesive was also developed using a resin of PMGDM, EBPADMA, 2-hydroxyethyl methacrylate (HEMA), and bisphenol A glycidyl dimethacrylate (BisGMA), which was referred to as PEHB120. After being recharged, the ion-exhausted PEHB+NACP adhesive continuously re-released Ca and P ions for 2–3 weeks121. Therefore, the rechargeable EBPM+NACP composite and PEHB+NACP adhesive provided the possibility for long-term remineralization.
A recent study demonstrated the long-term remineralization of PAMAM+NACP for the first time122. First, demineralized dentin was coated with PAMAM, and then immersed in a phosphate-buffered saline (PBS, pH 7.4) with a constant severe shaking for 72 days (referred to as the immersed-PAMAM-coated dentin). This action simulated saliva flow and drinking which could potentially detached the coated PAMAM. Separately, EBPM+NACP composite was immersed in a pH 4 lactic acid solution for 72 days to completely exhaust the Ca and P ion release. Then, the ion-exhausted EBPM+NACP composite was recharged with a Ca and P ion solution simulating a mouthwash. The EBPM+NACP composite was then placed in contact with the immersed-PAMAM-coated dentin, and the construct was treated with a cyclic remineralization/demineralization regimen for 35 days. The results showed that most of the PAMAM molecules were still attached on the demineralized dentin after 72 days of fluid shaking challenge. The recharged EBPM+NACP composites re-released Ca and P ions, and the ion-release and acid-neutralization capacity did not decrease with increasing the number of release-recharge cycles (Fig. 7). As a result, the immersed-PAMAM and recharged EBPM+NACP composite achieved triple benefits: stable nucleation templates, constant Ca and P ion release, and continuous acid-neutralization. The combination yielded the greatest mineral precipitation both in the dentinal tubules and on the dentin surface (Fig. 8)122. In addition, the hardness of the pre-demineralized dentin was increased to that of healthy dentin. Therefore, the PAMAM+NACP strategy is promising to provide long-term tooth-protection and caries-inhibition effects. Further studies are needed to investigate the remineralization durability of PAMAM+NACP for longer terms, for example, for 6 months and 12 months. In addition, applying the PAMAM solution before placing the NACP composite and adhesive may affect the bonding strength and decrease the long-term remineralization effect. Further study is needed to determine whether the application of PAMAM would negatively affect the bonding strength of NACP composite and adhesive.
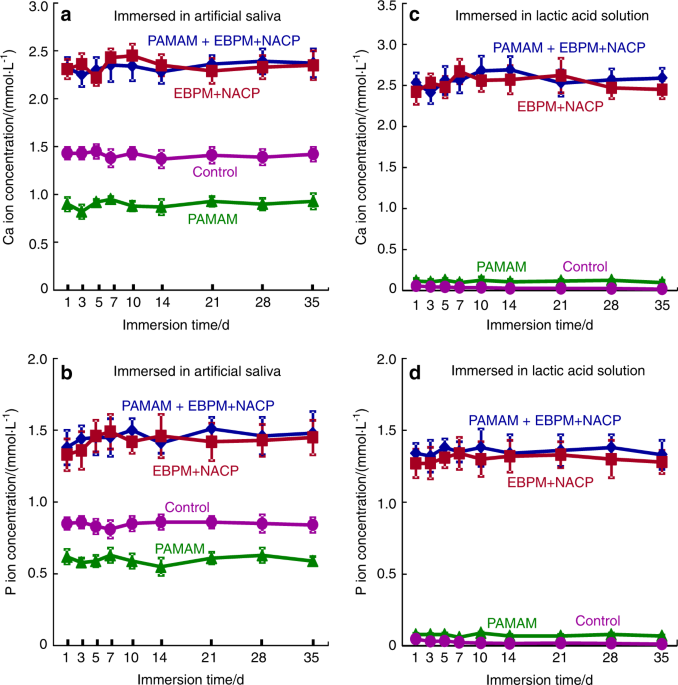
(a, b) Ca and P ion concentrations of artificial saliva and (c, d) lactic acid. EBPM+NACP group and PAMAM+EBPM+NACP group had higher Ca and P concentrations than control and PAMAM groups. PAMAM group had lower ion concentrations in artificial saliva than control group, indicating that PAMAM promoted Ca and P ion precipitation in demineralized dentin during artificial saliva immersion. For EBPM+NACP and PAMAM+EBPM+NACP groups, the ion concentrations did not decrease with repeated recharge, even though a fresh solution was used to immerse the samples every day. (Adapted from Liang et al.122 with permission.)
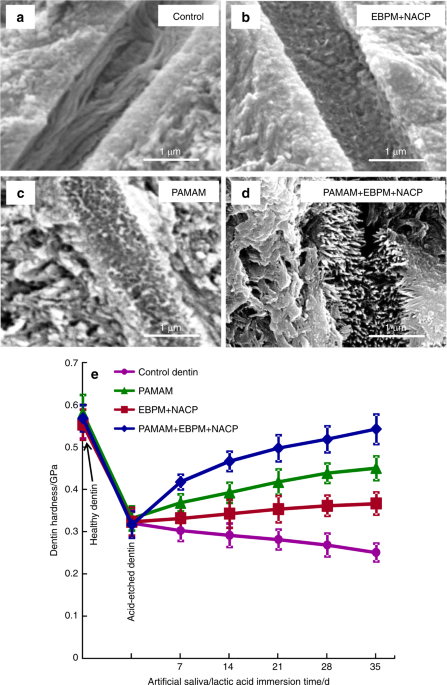
SEM micrographs of demineralized dentin cross-sections parallel to the dentinal tubule axis after 35 days cyclic artificial saliva/lactic acid regimen: (a) control group, (b) EBPM+NACP, (c) PAMAM group, and (d) PAMAM+EBPM+NACP. (e) Dentin hardness. The hardness of healthy dentin, dentin after being acid-etched, and demineralized dentin after 7, 14, 21, 28, and 35 days of artificial saliva/lactic acid cyclic treatments for: control, PAMAM group, EBPM+NACP group, and PAMAM+EBPM+NACP group (mean ± SD; n = 6). Images were taken on dentin cross-sections in a subsurface region of 2–30 μm beneath the surface. In (a), tubules were full of exposed collagen fibrils. b and c shows mineral deposit in tubules due to remineralization. In (d), tubules were completely occluded by dense minerals. (e) PAMAM+NACP returned the hardness of demineralized dentin to the level of healthy dentin (P > 0.1). (Adapted from Liang et al.122 with permission.)
Conclusions
This article represents the first review on the combination of PAMAM with calcium phosphate nanoparticles to achieve bioactive and therapeutic properties to inhibit caries. PAMAM and NACP together achieved synergy and yielded triple benefits: excellent nucleation template, superior acid-neutralization, and Ca and P ion release. Even in an acidic pH 4 solution without any initial Ca and P ions, or after a long period of fluid flow challenge, the novel PAMAM+NACP method still induced complete dentin remineralization and increased the hardness of pre-demineralized dentin back to the hardness of healthy dentin. Therefore, the novel PAMAM+NACP strategy is promising to inhibit demineralization and provide long-term remineralization to inhibit caries and protect tooth structures.
References
Acknowledgements
This work was supported by National Natural Science Foundation of China (81670977, J.L. and 81800965, K.L.), Sichuan Science and Technology program (Grant no. 2017SZ0030), Fundamental Research Funds for Central University 2018SCU12016 (K.L.), China Postdoctoral Science Grant 2018M643507 (K.L.), Research Fund of West China Hospital WCHS-201705 (K.L.), Research Fund for Resins of Chinese Stomatological Association CSA-R2018-06 (K.L.), University of Maryland School of Dentistry bridging fund (H.H.K.X.), and University of Maryland Baltimore seed grant (H.H.K.X.).
Author information
Author notes
Affiliations
Competing interests
The authors declare no competing interests.
Corresponding authors
Correspondence to Jiyao Li or Hockin H. K. Xu.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου